V. Rositano, A. Gambini, P. Allegrini, A. Bernardi, L. Senaldi
Eur. J. Org. Chem. 2024, 27, e202301211
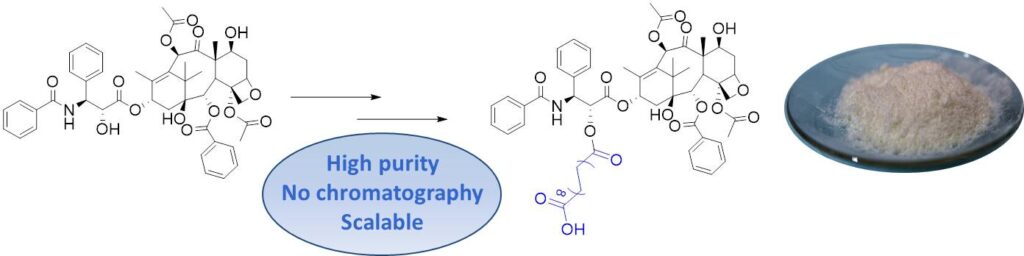
Paclitaxel is one of the most important chemotherapy agents and it has shown activity against a variety of human cancers. In the past years several derivatives and formulations have been developed to improve its activity and reduce its toxicity. Recently, 1,18-Octadecanedioic acid-Paclitaxel (ODDA-PTX), obtained by conjugation of paclitaxel with a long-chain diacid, has emerged as a promising paclitaxel prodrug, with high efficacy and low toxicity. A novel strategy for its synthesis has been developed and is herein described. The approach is based on the mono-allyl protection of octadecanedioic acid (ODDA) and on the Pd-catalyzed removal of the allyl ester after coupling with paclitaxel. This strategy is superior to previously reported procedures in that it affords the ODDA-PTX product with much higher purity, avoids chromatographic purifications and can be practically scaled-up for the industrial production of ODDA-PTX.