M. Mason, B. Nava, L. Belvisi, L. Pignataro, A. Dal Corso
EurJOC 2024, e202400229
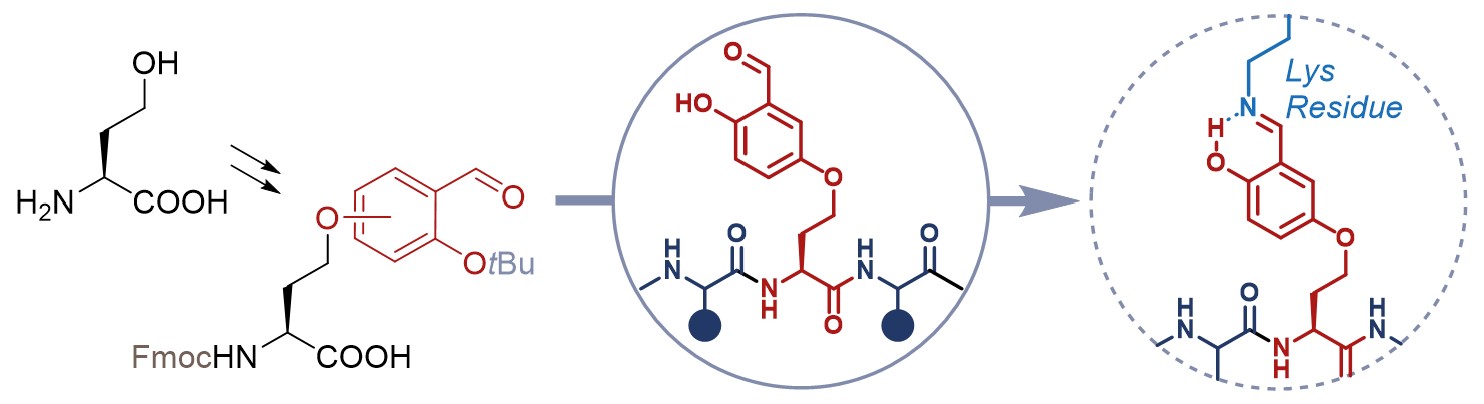
Insertion of electrophilic species on the structure of small molecule ligands or peptides is a well-known strategy to increase their binding affinity for the target protein of interest. Among these reactive units, the salicylaldehyde (SA) tag can form remarkably stable imine bonds with the ε-amino group of lysine, a highly frequent residue in proteins. In this work, we describe the optimized synthesis of two new noncoded α-amino acids, starting from L-homoserine and featuring the SA tag on the side chain. One of these final compounds was successfully inserted into a model tripeptide through in-solution synthesis. These building blocks will allow the versatile insertion of the SA tag at suitable position of peptide sequences, opening to a tailored design of Lys-engaging peptide ligands.